Volume 13, Issue 2 (2021)
Iran J War Public Health 2021, 13(2): 163-170 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2021/10/6 | Accepted: 2021/10/12 | Published: 2021/11/2
Received: 2021/10/6 | Accepted: 2021/10/12 | Published: 2021/11/2
How to cite this article
Shepetko-Dombrovska O, Varbanets S, Meshkova M, Mokhnatyi S, Marushko Y, Neshva V et al . Effectiveness of Left-Sided Surgical Ablation for Patients with Atrial Fibrillation Concomitant with Cardiac Surgery. Iran J War Public Health 2021; 13 (2) :163-170
URL: http://ijwph.ir/article-1-1038-en.html
URL: http://ijwph.ir/article-1-1038-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
O. Shepetko-Dombrovska *1, S. Varbanets1, M. Meshkova2, S. Mokhnatyi3, Y. Marushko4, V. Neshva1, N. Rudenko5
1- Department of Adult Cardiac Surgery, Clinic for Adults, Scientific-Practical Medical Center for Pediatric Cardiology and Cardiac Surgery, Kyiv, Ukraine
2- Department of Electrophysiology, Clinic for Adults, Scientific-Practical Medical Center for Pediatric Cardiology and Cardiac Surgery, Kyiv, Ukraine
3- Department of Emergency Surgery, Clinic for Adults, Scientific-Practical Medical Center for Pediatric Cardiology and Cardiac Surgery, Kyiv, Ukraine
4- Department of Interventional Cardiology, Clinic for Adults, Scientific-Practical Medical Center for Pediatric Cardiology and Cardiac Surgery, Kyiv, Ukraine
5- Department of Pediatric Cardiology and Cardiac Surgery, Clinic for Adults, Scientific-Practical Medical Center for Pediatric Cardiology and Cardiac Surgery, Kyiv, Ukraine
2- Department of Electrophysiology, Clinic for Adults, Scientific-Practical Medical Center for Pediatric Cardiology and Cardiac Surgery, Kyiv, Ukraine
3- Department of Emergency Surgery, Clinic for Adults, Scientific-Practical Medical Center for Pediatric Cardiology and Cardiac Surgery, Kyiv, Ukraine
4- Department of Interventional Cardiology, Clinic for Adults, Scientific-Practical Medical Center for Pediatric Cardiology and Cardiac Surgery, Kyiv, Ukraine
5- Department of Pediatric Cardiology and Cardiac Surgery, Clinic for Adults, Scientific-Practical Medical Center for Pediatric Cardiology and Cardiac Surgery, Kyiv, Ukraine
Full-Text (HTML) (525 Views)
Introduction
Atrial fibrillation (AF) is one of the most common heart rhythm disorders, affecting approximately 33.5 million worldwide [1]. At the moment, the frequency of atrial fibrillation occurs in almost 3% of the population of Western Europe aged 20 years and older. According to forecasts, by 2030, atrial fibrillation among European residents will increase from 7 million to almost 13 million [2]. Atrial fibrillation can be a complication of any pathology of the valvular heart apparatus. Mitral valve lesions, in particular in rheumatic heart disease, have the highest prevalence of AF. In patients with mild to moderate aortic stenosis, atrial fibrillation (AF) prevalence is 9.1% and 33.7% in those with severe aortic stenosis. Atrial fibrillation in patients with isolated mitral insufficiency develops in 16% of cases, with isolated mitral stenosis – in 29% of cases, and 52% with combined mitral defect (stenosis and regurgitation). The prevalence of AF reaches 70% with a combined mitral and tricuspid valve defect [3]. AF has some risk factors, such as hypertension, obesity, coronary heart disease, smoking, diabetes mellitus [4]. There are also risk factors that predispose to the progression of persistent AF in patients waiting for ablation.
Among them, hypertension, old age, ischemic attacks, and other conditions are distinguished [5]. Over the past decade, great progress has been made in identifying the genetic determinants of AF. Although studies of families with AF have led to the identification of mutations in several ion channels and molecules, these mutations are usually family-specific, rare, and do not explain a significant part of the inheritance of AF [6, 7]. AF is largely a disease of the elderly. Over the past decade, many studies have been conducted, in which special attention was paid to reporting the results of AF ablation in older adults [7-9].
Currently, it is extremely important to develop effective and safe treatment strategies to prevent or reverse the occurrence of AF in patients. Thus, most conventional antiarrhythmic drugs are contraindicated in heart failure and AF patients, often ineffective or poorly tolerated, making AF ablation an increasingly important option for heart rate control [10]. Over the past three decades, catheter and surgical ablation of atrial fibrillation (AF) have evolved from examinational procedures to their current role as effective treatment options for patients with AF. Surgical ablation of AF using standard, minimally invasive, or hybrid methods are available in most major hospitals worldwide. Catheter ablation of AF has become even more accessible and is currently the most frequently performed catheter ablation procedure [7]. However, despite the considerable success of drug and surgical treatment of atrial fibrillation, choosing the optimal treatment for this arrhythmia remains a difficult problem to date. The severity of AF often has a direct connection with the occurrence of other pathologies. For example, emerging data have shown that permanent forms of AF are associated with a significant increase in thrombembolia and death compared to paroxysmal AF [11].
Modern treatment of AF includes the active use of surgical methods of treatment, which show high efficiency in restoring and maintaining the sinus rhythm [12, 13]. As a result of the study, the authors expect to cover the effectiveness of surgical ablation of the atrial fibrillation substrate during cardiac surgery.
Materials and Methods
In the period from January 1, 2016, to July 1, 2019, 46 procedures of left atrial surgical ablation of the atrial fibrillation substrate were performed in patients with paroxysmal, persistent, and long-lasting persistent AF based on the State Institution "Scientific and Practical Medical Centre of Pediatric Cardiology and Cardiac Surgery of the Ministry of Health of Ukraine". Equation of calculating sample size was n = [(Zα/2 + Zβ)2 × {2(ó)2}]/ (μ1-μ2)2. According to it, patients with a permanent form of AF who decided to restore the sinus rhythm were assigned to the main group of patients with a long-lasting persistent form of AF (N=46). For comparison, the authors also studied a control group of patients with atrial fibrillation who underwent surgery from January 1, 2016, to July 1, 2019, but, for some reason, these patients did not undergo left atrial surgical ablation of the atrial fibrillation substrate. The control group consisted of 23 studied patients. The patient groups were homogeneous and had approximately the same demographic indicators.
The anamnestic survey was performed during the study; additional data was gathered from medical cards and monitoring. This study was approved by the National Ethics Commission of the Ministry of Health of Ukraine. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Patients from the study groups underwent ECGpro Holter monitoring (IMESC, Ukraine), ECGpro 12-channel resting electrocardiography (IMESC, Ukraine), anamnestic survey 3, 6, 12, and 24 months after the surgical intervention. Patients from the control group also underwent electrocardiography and anamnestic survey. Some patients underwent daily Holter ECG monitoring.
During echocardiography and evaluation of the left atrium in all patients using the transthoracic echocardiography (TTE) technique, the anterior-posterior size of the left atrium was measured in the 2D model in the middle of the systole. TTE was performed using a Philips IE-33 device (Providian medical, Ukraine) with an S5-1 sensor or a Philips Cx-50. Transthoracic echocardiography was performed according to the recommendations of the American Association of Echocardiography [12]. Attention was focused on visualization of the left atrium and measurement of its size. The procedure of the atrial fibrillation substrate's left atrial radiofrequency surgical ablation was performed together with surgical intervention for valvular pathology, congenital malformations (atrial septal defect, cor triatriatum), and coronary artery bypass grafting. The procedure of surgical ablation of the atrial fibrillation substrate with a unipolar radiofrequency electrode was performed under conditions of artificial blood circulation. All patients underwent median sternotomy. All patients underwent only left-sided surgical ablation, ablator-IBI-1500 (Irvine Biomedical; USA). After accessing the mitral valve using a monopolar electrode, IBI Therapy™ CooI Path™ Ablation Catheter, St. Jude Medical, the USA with active cooling, lines were applied on the inner surface of the left atrium. Firstly, lines were drawn around the left and right pulmonary veins forming a closed circle. Then both circles were connected with an additional line, preventing omissions. In addition, a line was applied from the left atrial (LA) appendage to the previously applied lines.
Ablation was completed by applying a line to the mitral valve in the direction of the F2 segment (Figure 1).

Figure 1) Scheme of left atrial surgical ablation of the atrial fibrillation substrate
The whole process was controlled by an arrhythmologist to avoid overheating the electrode and incomplete contact with the LA wall. After the surgery, all patients received Class I/III antiarrhythmic drugs. Patients with coronary heart disease received beta-blockers at a dose of 2.5-5mg and additionally, amiodarone 200mg 3 times a day before a saturation dose of 10g, then a maintenance dose of amiodarone: 200mg 1 time a day under the control of heart rate for 3-6 months. After the sinus rhythm was restored, antiarrhythmic drugs were canceled after 3-6 months. Catamnestic data were collected prospectively in all patients with surgical ablation of the AF substrate. It was not possible to collect data from 2 patients, as contact with them was lost. Further observation of all other (44 of patients) was carried out by daily Holter ECG monitoring, electrocardiogram, and anamnestic survey 3, 6, 12 months after the procedure, then annually.
The ANOVA and independent t-test were used to check the difference between groups. Graphpad prims was used as an analysis solution.
Findings
The clinical characteristics of the patients are demonstrated in Table 1. the majority of patients were men (60.9%), and there were 18 women (39.2%). The average observation terms of the comparative groups were 24 months (from 8 to 49 months). The patient groups were homogeneous in almost all parameters. A considerable difference was present only in the indicators of New York Heart Association Functional Classification class III (NYHA III) and the long-lasting persistent form of AF. This is due to a significantly smaller number of patients with these indicators in the control group.
Table 1) Clinical characteristics of patients (р>0.05 for all)

The relationship between the size of the LA and the possible recurrence of atrial fibrillation is presented in Diagram 1.
The characteristics of surgical interventions performed with left atrial radiofrequency surgical ablation are shown in Table 2. Most often, this procedure was performed together with prosthesis or mitral valve plasty.

Diagram 1) The relationship between the size of the left atrium and the possible recurrence of atrial fibrillation
Table 2) Characteristics of cardiac surgical interventions that were performed along with left atrial radiofrequency surgical ablation of the atrial fibrillation substrate (N=44)

Forty-three patients from the main group underwent resection of the left atrial appendage. On average, the aortic cross-clamp time in the main group (n=46) was 133±39.48 minutes.
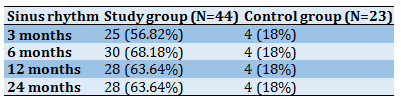
The data of patients on the restoration of sinus rhythm are presented in Table 3. The majority of patients experienced recovery and retention of the sinus rhythm during the entire time after the surgical intervention. Twenty-five patients (56.8%) retained a sinus rhythm after three months, 30 patients (68.2%) after six months, 28 patients (63.6%)after 12 months. 7 patients (15.9%) have a persistent AF, five patients (11.4%) have a typical atrial flutter, one patient (2.2%) was diagnosed with a rhythm disorder in the form of left atrial flutter, one patient (2.2%) with a persistent multi-focal atrial tachycardia. During the first 3-6 months after the procedure, some patients have recurrences of AF. 15% of patients require restoration of the sinus rhythm by electro cardioversion and consultation of an arrhythmology-electrophysiologist to select antiarrhythmic therapy.
Table 3) Characteristics of heart rate in patients after left-sided surgical ablation of the AF substrate and data on patients in the control group (р<0.001 for all)
Discussion
Left atrial flutter most often occurs after catheter or surgical ablation of atrial fibrillation and in patients with atrial myopathies [14]. Left atrial flutter is much less common than right atrial CTI-dependent flutter. As a rule, it is registered in patients with an organic lesion of the left parts of the heart. The basis for the development of macro reentry in LA are zones of delayed conduction or blockage of the impulse, which can be detected during its endocardial mapping. The following regions of LA most often serve as sources of fluttering: the area of the mitral valve, pulmonary veins, the posterior wall of the LA, the atrial septum [15]. After a successful procedure of left atrial surgical ablation of the atrial fibrillation substrate, provided that the sinus rhythm is steadily restored, as well as if the patient has undergone cardiac surgery without the use of mechanical prostheses, it is possible to recommend the abolition of anticoagulants after 3-6 months.
An important aspect is that the frequency of implantation of a cardiostimulator is significantly lower with left atrial ablation. Out of the 44 patients studied, there were no patients who needed cardiostimulator implantation. The following results were obtained were evaluating the postsurgical results of patients with cardiac surgery who did not undergo the procedure of left atrial surgical ablation of the atrial fibrillation substrate. Out of 23 patients with atrial fibrillation who underwent surgery, after eliminating the valvular defect, the sinus rhythm was restored and preserved during the entire follow-up period only in 4 (18%) patients. All other patients, namely 19 (82%), have a persistent or permanent form of AF. Thus, in the study group, the procedure's success was achieved in most patients, and ablation of the atrial fibrillation substrate during cardiac surgery effectively reduced the symptoms of atrial fibrillation. The opposite situation was in the group of patients who did not undergo ablation.
The main disadvantage of surgical ablation studies is that the literature is heterogeneous with inconsistencies in the types of patients studied, indications, sets of lesions, energy devices used, and, most importantly, the definitions of results [16]. In patients who, for some reason, did not undergo this procedure, the sinus rhythm was restored independently only in isolated cases. There is a rhythm disturbance in the form of atrial fibrillation in such patients, which requires further measures to restore the sinus rhythm.
The gold standard for surgical ablation of the atrial fibrillation substrate is the Cox-Maze procedure. More than 30 years have passed since it was performed for the first time in 1987, the surgical treatment of patients with AF has made considerable progress [17]. This is the most effective procedure: the sinus rhythm is restored in almost 97% of patients [18]. According to the literature, there are only three main methods for the correct implementation of the Cox-Maze procedure: (I) this is a "cut-and-sew" with cryoablation around critical zones (coronary sinus, mitral, and tricuspid rings), (II) cryoablation as an independent procedure and (III) bipolar radiofrequency ablation in combination with cryoablation around critical zones [19]. Surgical treatment of atrial fibrillation has undergone many changes. Cox-Maze IV helped to solve some of the limitations of Cox-Maze III. Using ablation technology to replace surgical incisions, similar cure rates were achieved, despite stricter follow-up criteria. Owing to the advances in mini-invasive technologies, Cox-Maze IV surgery can now be performed using a less invasive approach in correctly selected patients, resulting in a shorter hospital stay with equivalent 2-year results. As the mechanisms of AF become more understood and technologies continue to improve, surgical treatment methods are likely to become less invasive with improved cure rates since the procedures are adapted to the specific pathology of the patient [20].
A recent paper [17] reviewed the indications and presurgical planning for Cox-Maze IV, described the surgical technique, and reviewed the literature, including comparing Cox-Maze IV with the previous method. This review also investigated the future areas of surgical treatment of patients with AF. The authors considered whether Cox-Maze IV is still the gold standard and concluded that the main attention of doctors should be focused on training patients and surgeons for more aggressive treatment of concomitant AF. The authors note that as people learn more about the mechanisms of AF and develop improved presurgical diagnostic technologies that can accurately identify the mechanisms, it may be possible to adapt specific ablation techniques and methods to individual patients, which will make surgical treatment of AF more effective and accessible to even more patients. In the Centre of Pediatric Cardiology and Cardiac Surgery in the clinic for adults, only left-sided ablation of the atrial fibrillation substrate with a unipolar radiofrequency electrode was performed. This procedure is not the gold standard of surgical ablation and is not as effective as Cox-Maze III or Cox-Maze IV, but there are significantly fewer surgical complications when performing this procedure.
The question of the need for more aggressive ablation during cardiac surgery is increasingly being raised. Thus, with the introduction of the Maze IV procedure using ablation in 2004, both the complexity of the surgery and the time required to complete a full set of lesions were sharply reduced, and no significant differences in morbidity or mortality were found among low, medium, and high-risk patients. In studies examining the database of the Society of Thoracic Surgeons, only about 20% to 39% of patients with atrial fibrillation had surgical ablation performed during the surgery [21]. Atrial fibrillation is often found in patients undergoing mitral valve surgery. Recent data show that AF ablation improves outcomes for these patients. Thus, almost all such AF patients need to undergo mitral valve surgery and AF ablation [22].
The procedure of left atrial surgical ablation of the atrial fibrillation substrate should be carried out taking into account the individual parameters of each patient (the size of the left atrium, the left ventricular ejection fraction, hemodynamically significant, and producing symptoms arrhythmia should be taken into account). It is important to choose the method of surgical intervention. For example, mechanical valve prostheses require a lifelong intake of anticoagulants (vitamin K antagonists). Therefore, it should be borne in mind that in such cases, if the arrhythmia does not cause any symptoms and the heart rate is well controlled by antiarrhythmic drugs, the benefits of the procedure are debatable. The frequent need for repeated ablation and limited overall success rates are still the main limitations of catheter ablation procedures for treating atrial fibrillation (AF). In the future, the substrate modification during AF ablation should switch to individualized ablation procedures adapted to the individual needs of the patient [23].
The effectiveness and safety of simplified surgical ablation of the left atrium have not been established; there have been separate articles on this topic for a long time. Meta-analysis of RCCT [24] identified acceptable 30-day mortality (range: 0–8.3%) and levels of late mortality from all causes (range: 0–7.5%) in the included RCCT without a significant difference between the groups of patients with surgical intervention with left atrial ablation and without. However, a small number of patients in the RCCT showed that this result should be treated with caution.
The current randomized data do not demonstrate a clinically significant reduction in the risk of stroke due to AF ablation. There are many reasons for this. Firstly, the total stroke rate is very low in modern populations with AF subjected to appropriate anticoagulation; therefore, even a considerable relative reduction in the risk of stroke will still lead to a small net benefit. Secondly, the residual risk of stroke may be largely associated with
noncardioembolic stroke, in which a positive effect from AF ablation cannot be expected. Thirdly, even occasional short, potentially silent recurrences of AF after successful AF ablation may have consequences for stroke risk. Finally, adverse atrial remodeling (atrial cardiopathy) might be the dominant risk factor for stroke, which is relatively unaffected by ablation. A relatively understudied area is the problem of hidden cerebral microembolism with concomitant cognitive impairment, and further research should focus on whether AF ablation has any long-term effect in this regard. Pragmatic discussions about the long-term significance of AF ablation should move away from the stroke-oriented approach and focus on the potential reduction of cardiovascular mortality and hospitalization (for which there is more encouraging data) [6].
Important criteria for unfavorable results are the patient's age over 75 years, the left atrium size of 5cm or more, the persistence of atrial fibrillation for more than 5 years, and the persistent and long-lasting persistent form of atrial fibrillation. The most favorable outcome for treatment is the paroxysmal form of atrial fibrillation [25]. According to the literature, patients who have two or more criteria for an unfavorable outcome of surgical ablation have the following results: after six months, a sinus rhythm was registered in 66% of patients, after 12 months (in 76% of patients, after two years) 70% of patients had a sinus rhythm [25]. The left atrium size was 51±6.12mm in the study group and 52±6.97mm in the control group. A small percentage (8.7%) of the studied patients had a paroxysmal form of atrial fibrillation (only 4). A fairly large number of patients (26 cases) had a long-term persistent form of atrial fibrillation (56.5%). A rather heterogeneous group of patients was used for surgical ablation of the AF substrate, significantly affecting the result.
Obesity is the main risk factor for atrial fibrillation. It also affects the natural course of the disease, leading to more persistent forms and worsening of ablation results. To improve outcomes in obese patients who have undergone AF ablation, a preliminary quantitative assessment of epicardial adipose tissue can help identify patients with an increased risk of recurrence. Implementing measures to reduce this risk through weight loss strategies and potential specific pharmacotherapy can effectively provide the best chance of success in this cohort. As for the procedure, some measures can be taken to reduce the risk of complications associated with ablation, and methods aimed at isolating the posterior wall of the left atrium should be considered. Given the fact that the success of ablation is determined not only by the technique but also by the characteristics of the patient, it is important to understand better the pathophysiological mechanism linking obesity and AF since this can pave the way to new therapeutic goals and improve patient treatment outcomes [26].
The study of the medical literature allows identifying some special studies devoted to the effectiveness of surgical ablation of the atrial fibrillation substrate during cardiac surgery. There is also a consensus of experts from the world's leading medical communities [7, 27], which extensively discusses catheter use and surgical ablation of atrial fibrillation. Approaches to surgical ablation, rationality, and effectiveness of its use for a group of patients with AF and heart failure (HF) are considered in a recent review [10, 28]. The authors note that, given the limited treatment options for AF for a group of patients with concomitant HF, catheter ablation provides a better approach to rhythm and symptom control than drug therapy and leads to considerable clinical and functional improvements.
The relationship between AF and CH is complex. Data confirm the connection of earlier AF ablation and improved treatment results. There is an opinion that doctors may consider lower requirements for referring patients with AF and HF for catheter ablation surgery. Based on the new recommendations, even for patients in whom AF may not cause symptoms, ablation may be explained due to subsequent clinical adverse outcomes [27-29]. Even though the number of deaths associated with the procedure during hospitalization after catheter ablation of AF was low, adverse outcomes may occur after discharge. Postsurgical complications, congestive heart failure, and a low volume of AF ablation in the hospital were predictors of early mortality. Timely treatment of postsurgical complications and CHF can be crucial for reducing mortality rates after AF ablation. During the follow-up period, no deaths from any causes were observed after ablation in the patients from the studied group.
Ablation in the treatment of AF can be performed by different methods. In this paper, the authors investigated the surgical ablation of the atrial fibrillation substrate in cardiac surgery. Based on the obtained data, it can be concluded that the effectiveness of this procedure was confirmed. In most patients, recovery and retention of the sinus rhythm were observed throughout the entire time after the surgical intervention.
The procedure of left atrial surgical ablation of the AF substrate is advisable for patients with atrial fibrillation since, in almost 65% of cases, the procedure provides restoration and preservation of the sinus rhythm. This considerably improves the quality of patients' life and allows them to cancel drug therapy with anticoagulants in the future (after 3-6 months). When referring patients with cardiac pathology and concomitant AF for cardiac surgery, the question of ablation during this intervention should be considered. It is extremely important to raise the awareness of treating doctors about this. The tactics of managing a patient with atrial fibrillation should be decided on an individual basis. The strategy for each case should consider the patient's general prognosis, age, concomitant pathology, and treatment goals.
The study's practical significance was to substantiate the rationality and effectiveness of the use of surgical ablation of the atrial fibrillation substrate in cardiac surgery. The materials of this paper may be useful for doctors and researchers whose activities are related to the development of treatment methods and the treatment of patients with AF itself.
Conclusion
In the course of the research, new questions and problems arose that needed to be solved. This study demonstrates effectiveness on a limited sample. The results of large-scale randomized research involving a larger number of patients, the presence of solid endpoints (hospitalization, fatal case), and a longer follow-up period will give a definite answer to the question of how effective and safe surgical ablation of the atrial fibrillation substrate during cardiac surgery is by the method used in this study in terms of clinical results, improving the quality of life and its duration. It is necessary to continue working on the development of surgical ablation techniques. The possibility of improving ablation techniques and improving the understanding of the origin and pathophysiology of AF will positively impact the quality and expectancy of life of patients with atrial fibrillation.
Acknowledgments: There is nothing to be declared.
Ethical Permissions: The study was approved by the National Ethics Commission of the Ministry of Health of Ukraine on September 01, 2021, No 0901-1.
Conflicts of Interests: There is nothing to be declared.
Authors' Contribution: Shepetko-Dombrovska O. (First Author), Methodologist (20%); Varbanets S. (Second Author), Introduction Writer/Methodologist (15%); Meshkova M. (Third Author), Assistant Researcher (15%); Mokhnatyi S. (Fourth Author), Introduction Writer/Methodologist (15%); Marushko Y. (Fifth Author), Assistant Researcher/Discussion Writer (15%); Neshva V. (Sixth Author), Data Analyst (10%); Rudenko N. (Seventh Author), Data Analyst/Discussion Writer (10%)
Funding/Support: There is nothing to be declared.
Atrial fibrillation (AF) is one of the most common heart rhythm disorders, affecting approximately 33.5 million worldwide [1]. At the moment, the frequency of atrial fibrillation occurs in almost 3% of the population of Western Europe aged 20 years and older. According to forecasts, by 2030, atrial fibrillation among European residents will increase from 7 million to almost 13 million [2]. Atrial fibrillation can be a complication of any pathology of the valvular heart apparatus. Mitral valve lesions, in particular in rheumatic heart disease, have the highest prevalence of AF. In patients with mild to moderate aortic stenosis, atrial fibrillation (AF) prevalence is 9.1% and 33.7% in those with severe aortic stenosis. Atrial fibrillation in patients with isolated mitral insufficiency develops in 16% of cases, with isolated mitral stenosis – in 29% of cases, and 52% with combined mitral defect (stenosis and regurgitation). The prevalence of AF reaches 70% with a combined mitral and tricuspid valve defect [3]. AF has some risk factors, such as hypertension, obesity, coronary heart disease, smoking, diabetes mellitus [4]. There are also risk factors that predispose to the progression of persistent AF in patients waiting for ablation.
Among them, hypertension, old age, ischemic attacks, and other conditions are distinguished [5]. Over the past decade, great progress has been made in identifying the genetic determinants of AF. Although studies of families with AF have led to the identification of mutations in several ion channels and molecules, these mutations are usually family-specific, rare, and do not explain a significant part of the inheritance of AF [6, 7]. AF is largely a disease of the elderly. Over the past decade, many studies have been conducted, in which special attention was paid to reporting the results of AF ablation in older adults [7-9].
Currently, it is extremely important to develop effective and safe treatment strategies to prevent or reverse the occurrence of AF in patients. Thus, most conventional antiarrhythmic drugs are contraindicated in heart failure and AF patients, often ineffective or poorly tolerated, making AF ablation an increasingly important option for heart rate control [10]. Over the past three decades, catheter and surgical ablation of atrial fibrillation (AF) have evolved from examinational procedures to their current role as effective treatment options for patients with AF. Surgical ablation of AF using standard, minimally invasive, or hybrid methods are available in most major hospitals worldwide. Catheter ablation of AF has become even more accessible and is currently the most frequently performed catheter ablation procedure [7]. However, despite the considerable success of drug and surgical treatment of atrial fibrillation, choosing the optimal treatment for this arrhythmia remains a difficult problem to date. The severity of AF often has a direct connection with the occurrence of other pathologies. For example, emerging data have shown that permanent forms of AF are associated with a significant increase in thrombembolia and death compared to paroxysmal AF [11].
Modern treatment of AF includes the active use of surgical methods of treatment, which show high efficiency in restoring and maintaining the sinus rhythm [12, 13]. As a result of the study, the authors expect to cover the effectiveness of surgical ablation of the atrial fibrillation substrate during cardiac surgery.
Materials and Methods
In the period from January 1, 2016, to July 1, 2019, 46 procedures of left atrial surgical ablation of the atrial fibrillation substrate were performed in patients with paroxysmal, persistent, and long-lasting persistent AF based on the State Institution "Scientific and Practical Medical Centre of Pediatric Cardiology and Cardiac Surgery of the Ministry of Health of Ukraine". Equation of calculating sample size was n = [(Zα/2 + Zβ)2 × {2(ó)2}]/ (μ1-μ2)2. According to it, patients with a permanent form of AF who decided to restore the sinus rhythm were assigned to the main group of patients with a long-lasting persistent form of AF (N=46). For comparison, the authors also studied a control group of patients with atrial fibrillation who underwent surgery from January 1, 2016, to July 1, 2019, but, for some reason, these patients did not undergo left atrial surgical ablation of the atrial fibrillation substrate. The control group consisted of 23 studied patients. The patient groups were homogeneous and had approximately the same demographic indicators.
The anamnestic survey was performed during the study; additional data was gathered from medical cards and monitoring. This study was approved by the National Ethics Commission of the Ministry of Health of Ukraine. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Patients from the study groups underwent ECGpro Holter monitoring (IMESC, Ukraine), ECGpro 12-channel resting electrocardiography (IMESC, Ukraine), anamnestic survey 3, 6, 12, and 24 months after the surgical intervention. Patients from the control group also underwent electrocardiography and anamnestic survey. Some patients underwent daily Holter ECG monitoring.
During echocardiography and evaluation of the left atrium in all patients using the transthoracic echocardiography (TTE) technique, the anterior-posterior size of the left atrium was measured in the 2D model in the middle of the systole. TTE was performed using a Philips IE-33 device (Providian medical, Ukraine) with an S5-1 sensor or a Philips Cx-50. Transthoracic echocardiography was performed according to the recommendations of the American Association of Echocardiography [12]. Attention was focused on visualization of the left atrium and measurement of its size. The procedure of the atrial fibrillation substrate's left atrial radiofrequency surgical ablation was performed together with surgical intervention for valvular pathology, congenital malformations (atrial septal defect, cor triatriatum), and coronary artery bypass grafting. The procedure of surgical ablation of the atrial fibrillation substrate with a unipolar radiofrequency electrode was performed under conditions of artificial blood circulation. All patients underwent median sternotomy. All patients underwent only left-sided surgical ablation, ablator-IBI-1500 (Irvine Biomedical; USA). After accessing the mitral valve using a monopolar electrode, IBI Therapy™ CooI Path™ Ablation Catheter, St. Jude Medical, the USA with active cooling, lines were applied on the inner surface of the left atrium. Firstly, lines were drawn around the left and right pulmonary veins forming a closed circle. Then both circles were connected with an additional line, preventing omissions. In addition, a line was applied from the left atrial (LA) appendage to the previously applied lines.
Ablation was completed by applying a line to the mitral valve in the direction of the F2 segment (Figure 1).

Figure 1) Scheme of left atrial surgical ablation of the atrial fibrillation substrate
The whole process was controlled by an arrhythmologist to avoid overheating the electrode and incomplete contact with the LA wall. After the surgery, all patients received Class I/III antiarrhythmic drugs. Patients with coronary heart disease received beta-blockers at a dose of 2.5-5mg and additionally, amiodarone 200mg 3 times a day before a saturation dose of 10g, then a maintenance dose of amiodarone: 200mg 1 time a day under the control of heart rate for 3-6 months. After the sinus rhythm was restored, antiarrhythmic drugs were canceled after 3-6 months. Catamnestic data were collected prospectively in all patients with surgical ablation of the AF substrate. It was not possible to collect data from 2 patients, as contact with them was lost. Further observation of all other (44 of patients) was carried out by daily Holter ECG monitoring, electrocardiogram, and anamnestic survey 3, 6, 12 months after the procedure, then annually.
The ANOVA and independent t-test were used to check the difference between groups. Graphpad prims was used as an analysis solution.
Findings
The clinical characteristics of the patients are demonstrated in Table 1. the majority of patients were men (60.9%), and there were 18 women (39.2%). The average observation terms of the comparative groups were 24 months (from 8 to 49 months). The patient groups were homogeneous in almost all parameters. A considerable difference was present only in the indicators of New York Heart Association Functional Classification class III (NYHA III) and the long-lasting persistent form of AF. This is due to a significantly smaller number of patients with these indicators in the control group.
Table 1) Clinical characteristics of patients (р>0.05 for all)

The relationship between the size of the LA and the possible recurrence of atrial fibrillation is presented in Diagram 1.
The characteristics of surgical interventions performed with left atrial radiofrequency surgical ablation are shown in Table 2. Most often, this procedure was performed together with prosthesis or mitral valve plasty.

Diagram 1) The relationship between the size of the left atrium and the possible recurrence of atrial fibrillation
Table 2) Characteristics of cardiac surgical interventions that were performed along with left atrial radiofrequency surgical ablation of the atrial fibrillation substrate (N=44)

Forty-three patients from the main group underwent resection of the left atrial appendage. On average, the aortic cross-clamp time in the main group (n=46) was 133±39.48 minutes.
The data of patients on the restoration of sinus rhythm are presented in Table 3. The majority of patients experienced recovery and retention of the sinus rhythm during the entire time after the surgical intervention. Twenty-five patients (56.8%) retained a sinus rhythm after three months, 30 patients (68.2%) after six months, 28 patients (63.6%)after 12 months. 7 patients (15.9%) have a persistent AF, five patients (11.4%) have a typical atrial flutter, one patient (2.2%) was diagnosed with a rhythm disorder in the form of left atrial flutter, one patient (2.2%) with a persistent multi-focal atrial tachycardia. During the first 3-6 months after the procedure, some patients have recurrences of AF. 15% of patients require restoration of the sinus rhythm by electro cardioversion and consultation of an arrhythmology-electrophysiologist to select antiarrhythmic therapy.
Table 3) Characteristics of heart rate in patients after left-sided surgical ablation of the AF substrate and data on patients in the control group (р<0.001 for all)

Discussion
Left atrial flutter most often occurs after catheter or surgical ablation of atrial fibrillation and in patients with atrial myopathies [14]. Left atrial flutter is much less common than right atrial CTI-dependent flutter. As a rule, it is registered in patients with an organic lesion of the left parts of the heart. The basis for the development of macro reentry in LA are zones of delayed conduction or blockage of the impulse, which can be detected during its endocardial mapping. The following regions of LA most often serve as sources of fluttering: the area of the mitral valve, pulmonary veins, the posterior wall of the LA, the atrial septum [15]. After a successful procedure of left atrial surgical ablation of the atrial fibrillation substrate, provided that the sinus rhythm is steadily restored, as well as if the patient has undergone cardiac surgery without the use of mechanical prostheses, it is possible to recommend the abolition of anticoagulants after 3-6 months.
An important aspect is that the frequency of implantation of a cardiostimulator is significantly lower with left atrial ablation. Out of the 44 patients studied, there were no patients who needed cardiostimulator implantation. The following results were obtained were evaluating the postsurgical results of patients with cardiac surgery who did not undergo the procedure of left atrial surgical ablation of the atrial fibrillation substrate. Out of 23 patients with atrial fibrillation who underwent surgery, after eliminating the valvular defect, the sinus rhythm was restored and preserved during the entire follow-up period only in 4 (18%) patients. All other patients, namely 19 (82%), have a persistent or permanent form of AF. Thus, in the study group, the procedure's success was achieved in most patients, and ablation of the atrial fibrillation substrate during cardiac surgery effectively reduced the symptoms of atrial fibrillation. The opposite situation was in the group of patients who did not undergo ablation.
The main disadvantage of surgical ablation studies is that the literature is heterogeneous with inconsistencies in the types of patients studied, indications, sets of lesions, energy devices used, and, most importantly, the definitions of results [16]. In patients who, for some reason, did not undergo this procedure, the sinus rhythm was restored independently only in isolated cases. There is a rhythm disturbance in the form of atrial fibrillation in such patients, which requires further measures to restore the sinus rhythm.
The gold standard for surgical ablation of the atrial fibrillation substrate is the Cox-Maze procedure. More than 30 years have passed since it was performed for the first time in 1987, the surgical treatment of patients with AF has made considerable progress [17]. This is the most effective procedure: the sinus rhythm is restored in almost 97% of patients [18]. According to the literature, there are only three main methods for the correct implementation of the Cox-Maze procedure: (I) this is a "cut-and-sew" with cryoablation around critical zones (coronary sinus, mitral, and tricuspid rings), (II) cryoablation as an independent procedure and (III) bipolar radiofrequency ablation in combination with cryoablation around critical zones [19]. Surgical treatment of atrial fibrillation has undergone many changes. Cox-Maze IV helped to solve some of the limitations of Cox-Maze III. Using ablation technology to replace surgical incisions, similar cure rates were achieved, despite stricter follow-up criteria. Owing to the advances in mini-invasive technologies, Cox-Maze IV surgery can now be performed using a less invasive approach in correctly selected patients, resulting in a shorter hospital stay with equivalent 2-year results. As the mechanisms of AF become more understood and technologies continue to improve, surgical treatment methods are likely to become less invasive with improved cure rates since the procedures are adapted to the specific pathology of the patient [20].
A recent paper [17] reviewed the indications and presurgical planning for Cox-Maze IV, described the surgical technique, and reviewed the literature, including comparing Cox-Maze IV with the previous method. This review also investigated the future areas of surgical treatment of patients with AF. The authors considered whether Cox-Maze IV is still the gold standard and concluded that the main attention of doctors should be focused on training patients and surgeons for more aggressive treatment of concomitant AF. The authors note that as people learn more about the mechanisms of AF and develop improved presurgical diagnostic technologies that can accurately identify the mechanisms, it may be possible to adapt specific ablation techniques and methods to individual patients, which will make surgical treatment of AF more effective and accessible to even more patients. In the Centre of Pediatric Cardiology and Cardiac Surgery in the clinic for adults, only left-sided ablation of the atrial fibrillation substrate with a unipolar radiofrequency electrode was performed. This procedure is not the gold standard of surgical ablation and is not as effective as Cox-Maze III or Cox-Maze IV, but there are significantly fewer surgical complications when performing this procedure.
The question of the need for more aggressive ablation during cardiac surgery is increasingly being raised. Thus, with the introduction of the Maze IV procedure using ablation in 2004, both the complexity of the surgery and the time required to complete a full set of lesions were sharply reduced, and no significant differences in morbidity or mortality were found among low, medium, and high-risk patients. In studies examining the database of the Society of Thoracic Surgeons, only about 20% to 39% of patients with atrial fibrillation had surgical ablation performed during the surgery [21]. Atrial fibrillation is often found in patients undergoing mitral valve surgery. Recent data show that AF ablation improves outcomes for these patients. Thus, almost all such AF patients need to undergo mitral valve surgery and AF ablation [22].
The procedure of left atrial surgical ablation of the atrial fibrillation substrate should be carried out taking into account the individual parameters of each patient (the size of the left atrium, the left ventricular ejection fraction, hemodynamically significant, and producing symptoms arrhythmia should be taken into account). It is important to choose the method of surgical intervention. For example, mechanical valve prostheses require a lifelong intake of anticoagulants (vitamin K antagonists). Therefore, it should be borne in mind that in such cases, if the arrhythmia does not cause any symptoms and the heart rate is well controlled by antiarrhythmic drugs, the benefits of the procedure are debatable. The frequent need for repeated ablation and limited overall success rates are still the main limitations of catheter ablation procedures for treating atrial fibrillation (AF). In the future, the substrate modification during AF ablation should switch to individualized ablation procedures adapted to the individual needs of the patient [23].
The effectiveness and safety of simplified surgical ablation of the left atrium have not been established; there have been separate articles on this topic for a long time. Meta-analysis of RCCT [24] identified acceptable 30-day mortality (range: 0–8.3%) and levels of late mortality from all causes (range: 0–7.5%) in the included RCCT without a significant difference between the groups of patients with surgical intervention with left atrial ablation and without. However, a small number of patients in the RCCT showed that this result should be treated with caution.
The current randomized data do not demonstrate a clinically significant reduction in the risk of stroke due to AF ablation. There are many reasons for this. Firstly, the total stroke rate is very low in modern populations with AF subjected to appropriate anticoagulation; therefore, even a considerable relative reduction in the risk of stroke will still lead to a small net benefit. Secondly, the residual risk of stroke may be largely associated with
noncardioembolic stroke, in which a positive effect from AF ablation cannot be expected. Thirdly, even occasional short, potentially silent recurrences of AF after successful AF ablation may have consequences for stroke risk. Finally, adverse atrial remodeling (atrial cardiopathy) might be the dominant risk factor for stroke, which is relatively unaffected by ablation. A relatively understudied area is the problem of hidden cerebral microembolism with concomitant cognitive impairment, and further research should focus on whether AF ablation has any long-term effect in this regard. Pragmatic discussions about the long-term significance of AF ablation should move away from the stroke-oriented approach and focus on the potential reduction of cardiovascular mortality and hospitalization (for which there is more encouraging data) [6].
Important criteria for unfavorable results are the patient's age over 75 years, the left atrium size of 5cm or more, the persistence of atrial fibrillation for more than 5 years, and the persistent and long-lasting persistent form of atrial fibrillation. The most favorable outcome for treatment is the paroxysmal form of atrial fibrillation [25]. According to the literature, patients who have two or more criteria for an unfavorable outcome of surgical ablation have the following results: after six months, a sinus rhythm was registered in 66% of patients, after 12 months (in 76% of patients, after two years) 70% of patients had a sinus rhythm [25]. The left atrium size was 51±6.12mm in the study group and 52±6.97mm in the control group. A small percentage (8.7%) of the studied patients had a paroxysmal form of atrial fibrillation (only 4). A fairly large number of patients (26 cases) had a long-term persistent form of atrial fibrillation (56.5%). A rather heterogeneous group of patients was used for surgical ablation of the AF substrate, significantly affecting the result.
Obesity is the main risk factor for atrial fibrillation. It also affects the natural course of the disease, leading to more persistent forms and worsening of ablation results. To improve outcomes in obese patients who have undergone AF ablation, a preliminary quantitative assessment of epicardial adipose tissue can help identify patients with an increased risk of recurrence. Implementing measures to reduce this risk through weight loss strategies and potential specific pharmacotherapy can effectively provide the best chance of success in this cohort. As for the procedure, some measures can be taken to reduce the risk of complications associated with ablation, and methods aimed at isolating the posterior wall of the left atrium should be considered. Given the fact that the success of ablation is determined not only by the technique but also by the characteristics of the patient, it is important to understand better the pathophysiological mechanism linking obesity and AF since this can pave the way to new therapeutic goals and improve patient treatment outcomes [26].
The study of the medical literature allows identifying some special studies devoted to the effectiveness of surgical ablation of the atrial fibrillation substrate during cardiac surgery. There is also a consensus of experts from the world's leading medical communities [7, 27], which extensively discusses catheter use and surgical ablation of atrial fibrillation. Approaches to surgical ablation, rationality, and effectiveness of its use for a group of patients with AF and heart failure (HF) are considered in a recent review [10, 28]. The authors note that, given the limited treatment options for AF for a group of patients with concomitant HF, catheter ablation provides a better approach to rhythm and symptom control than drug therapy and leads to considerable clinical and functional improvements.
The relationship between AF and CH is complex. Data confirm the connection of earlier AF ablation and improved treatment results. There is an opinion that doctors may consider lower requirements for referring patients with AF and HF for catheter ablation surgery. Based on the new recommendations, even for patients in whom AF may not cause symptoms, ablation may be explained due to subsequent clinical adverse outcomes [27-29]. Even though the number of deaths associated with the procedure during hospitalization after catheter ablation of AF was low, adverse outcomes may occur after discharge. Postsurgical complications, congestive heart failure, and a low volume of AF ablation in the hospital were predictors of early mortality. Timely treatment of postsurgical complications and CHF can be crucial for reducing mortality rates after AF ablation. During the follow-up period, no deaths from any causes were observed after ablation in the patients from the studied group.
Ablation in the treatment of AF can be performed by different methods. In this paper, the authors investigated the surgical ablation of the atrial fibrillation substrate in cardiac surgery. Based on the obtained data, it can be concluded that the effectiveness of this procedure was confirmed. In most patients, recovery and retention of the sinus rhythm were observed throughout the entire time after the surgical intervention.
The procedure of left atrial surgical ablation of the AF substrate is advisable for patients with atrial fibrillation since, in almost 65% of cases, the procedure provides restoration and preservation of the sinus rhythm. This considerably improves the quality of patients' life and allows them to cancel drug therapy with anticoagulants in the future (after 3-6 months). When referring patients with cardiac pathology and concomitant AF for cardiac surgery, the question of ablation during this intervention should be considered. It is extremely important to raise the awareness of treating doctors about this. The tactics of managing a patient with atrial fibrillation should be decided on an individual basis. The strategy for each case should consider the patient's general prognosis, age, concomitant pathology, and treatment goals.
The study's practical significance was to substantiate the rationality and effectiveness of the use of surgical ablation of the atrial fibrillation substrate in cardiac surgery. The materials of this paper may be useful for doctors and researchers whose activities are related to the development of treatment methods and the treatment of patients with AF itself.
Conclusion
In the course of the research, new questions and problems arose that needed to be solved. This study demonstrates effectiveness on a limited sample. The results of large-scale randomized research involving a larger number of patients, the presence of solid endpoints (hospitalization, fatal case), and a longer follow-up period will give a definite answer to the question of how effective and safe surgical ablation of the atrial fibrillation substrate during cardiac surgery is by the method used in this study in terms of clinical results, improving the quality of life and its duration. It is necessary to continue working on the development of surgical ablation techniques. The possibility of improving ablation techniques and improving the understanding of the origin and pathophysiology of AF will positively impact the quality and expectancy of life of patients with atrial fibrillation.
Acknowledgments: There is nothing to be declared.
Ethical Permissions: The study was approved by the National Ethics Commission of the Ministry of Health of Ukraine on September 01, 2021, No 0901-1.
Conflicts of Interests: There is nothing to be declared.
Authors' Contribution: Shepetko-Dombrovska O. (First Author), Methodologist (20%); Varbanets S. (Second Author), Introduction Writer/Methodologist (15%); Meshkova M. (Third Author), Assistant Researcher (15%); Mokhnatyi S. (Fourth Author), Introduction Writer/Methodologist (15%); Marushko Y. (Fifth Author), Assistant Researcher/Discussion Writer (15%); Neshva V. (Sixth Author), Data Analyst (10%); Rudenko N. (Seventh Author), Data Analyst/Discussion Writer (10%)
Funding/Support: There is nothing to be declared.
Keywords:
References
1. Santos JV, Pereira J, Pinto R, Castro PM, Azevedo E, Freitas A. Atrial fibrillation as an ischemic stroke clinical and economic burden modifier: A 15-year nationwide study. Value Health. 2017;20(8):1083-91. [Link] [DOI:10.1016/j.jval.2017.04.018] [PMID]
2. Brandes A, Smit MD, Nguyen BO, Rienstra M, Van Gelder IC. Risk factor management in atrial fibrillation. Arrhythm Electrophysiol Rev. 2018;7(2):118-27. [Link] [DOI:10.15420/aer.2018.18.2] [PMID] [PMCID]
3. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114(9):1453-68. [Link] [DOI:10.1161/CIRCRESAHA.114.303211] [PMID]
4. Allan V. Are cardiovascular risk factors also associated with the incidence of atrial fibrillation? A systematic review and field synopsis of 23 factors in 32 population-based cohorts of 20 million participants. Thromb Haemost. 2017;117(5):837-50. [Link] [DOI:10.1160/TH16-11-0825] [PMID] [PMCID]
5. Kochhäuser S, Dechering DG, Trought K, Hache P, Haig-Carter T, Khaykin Y, et al. Predictors for progression of atrial fibrillation in patients awaiting atrial fibrillation ablation. Can J Cardiol. 2016;32(11):1348-54. [Link] [DOI:10.1016/j.cjca.2016.02.031] [PMID]
6. Christophersen IE, Ellinor PT. Genetics of atrial fibrillation: from families to genomes. J Hum Genet. 2016;61(1):61-70. [Link] [DOI:10.1038/jhg.2015.44] [PMID]
7. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-444. [Link]
8. Bunch TJ, May HT, Bair TL, Jacobs V, Crandall BG, Cutle M, et al. The impact of age on 5-year outcomes after atrial fibrillation catheter ablation. J Cardiovasc Electrophysiol. 2016;27(2):141-6. [Link] [DOI:10.1111/jce.12849] [PMID]
9. Metzner I. Ablation of atrial fibrillation in patients ≥75 years: long-term clinical outcome and safety. Europace. 2016;18(4):543-54. [Link] [DOI:10.1093/europace/euv229] [PMID]
10. Richter S, Di Biase L, Hindricks G. Atrial fibrillation ablation in heart failure. Eur Heart J. 2019;40(8):663-71. [Link] [DOI:10.1093/eurheartj/ehy778] [PMID]
11. Ganesan AN, Chew DP, Hartshorne T, Selvanayagam JB, Aylward PE, Sanders P, et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: A systematic review and meta-analysis. Eur Heart J. 2016;37(20):1591-602. [Link] [DOI:10.1093/eurheartj/ehw007] [PMID]
12. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J. 2015;16(3):233-71. [Link] [DOI:10.1093/ehjci/jev014] [PMID]
13. Damiano Jr RJ, Schwartz FH, Bailey MS, Maniar HS, Munfakh NA, Moon MR, et al. The Cox maze IV procedure: Predictors of late recurrence. J Thorac Cardiovasc Surg. 2011;141(1):113-21. [Link] [DOI:10.1016/j.jtcvs.2010.08.067] [PMID] [PMCID]
14. Markowitz SM, Thomas G, Liu CF, Cheung JW, Ip JE, Lerman BB. Approach to catheter ablation of left atrial flutters. J Cardiovasc Electrophysiol. 2019;30(12):3057-67. [Link] [DOI:10.1111/jce.14209] [PMID]
15. Lee KW, Yang Y, Scheinman MM. Atrial flutter: A review of its history, mechanisms, clinical features and current therapy. Curr Probl Cardiol. 2005;30(3):12-67. [Link] [DOI:10.1016/j.cpcardiol.2004.07.001] [PMID]
16. Ramlawi B, Abu Saleh WK. Surgical ablation of atrial fibrillation. Methodist Debakey Cardiovasc J. 2015;11(2):104-8. [Link] [DOI:10.14797/mdcj-11-2-104] [PMID] [PMCID]
17. Ruaengsri C, Schill MR, Khiabani AJ, Schuessler RB, Melby SJ, Damiano Jr RJ. The Cox-maze IV procedure in its second decade: Still the gold standard?. Eur J Cardiothorac Surg. 2018;53(suppl 1):i19-25. [Link] [DOI:10.1093/ejcts/ezx326] [PMID] [PMCID]
18. Ad N, Holmes SD, Lamont D, Shuman DJ. Left-sided surgical ablation for patients with atrial fibrillation who are undergoing concomitant cardiac surgical procedures. Ann Thorac Surg. 2017;103(1):58-65. [Link] [DOI:10.1016/j.athoracsur.2016.05.093] [PMID]
19. Lawrance CP, Henn MC, Damiano Jr RJ. Surgery for atrial fibrillation. Cardiol Clin. 2014;32(4):563-71. [Link] [DOI:10.1016/j.ccl.2014.07.003] [PMID] [PMCID]
20. Ad N. The Cox-Maze procedure: history, results, and predictors for failure. J Interv Card Electrophysiol. 2007;20(3):65-71. [Link] [DOI:10.1007/s10840-007-9176-z] [PMID]
21. Gillinov AM. Ablation of atrial fibrillation with mitral valve surgery. Curr Opin Cardiol. 2005;20(2):107-14. [Link] [DOI:10.1097/01.hco.0000153554.48122.86] [PMID]
22. Kottkamp H, Bender R, Berg J. Catheter ablation of atrial fibrillation: how to modify the substrate?. J Am Coll Cardiol. 2015;65(2):196-206. [Link] [DOI:10.1016/j.jacc.2014.10.034] [PMID]
23. Wang X, Wang C, Ye M, Lin J, Jin J, Hu Q, et al. Left atrial concomitant surgical ablation for treatment of atrial fibrillation in cardiac surgery: A meta-analysis of randomized controlled trials. PLoS One. 2018;13(1):e0191354. [Link] [DOI:10.1371/journal.pone.0191354] [PMID] [PMCID]
24. Barra S, Narayanan K, Boveda S, Primo J, Gonçalves H, Baran J, et al. Atrial fibrillation ablation and reduction of stroke events: Understanding the paradoxical lack of evidence. Stroke. 2019;50(10):2970-6. [Link] [DOI:10.1161/STROKEAHA.119.026890] [PMID]
25. Mangiafico V, Saberwal B, Lavalle C, Raharja A, Ahmed Z, Papageorgiou N, et al. Impact of obesity on atrial fibrillation ablation. Arch Cardiovasc Dis. 2020;113(8-9):551-63. [Link] [DOI:10.1016/j.acvd.2020.03.023] [PMID]
26. Aldaas OM, Malladi CL, Hsu JC. Catheter ablation of atrial fibrillation in patients with heart failure. Am J Cardiol. 2019;123(1):187-95. [Link] [DOI:10.1016/j.amjcard.2018.09.013] [PMID]
27. Cheng EP, Liu CF, Yeo I, Markowitz SM, Thomas G, Ip JE, et al. risk of mortality following catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2019;74(18):2254-64. [Link] [DOI:10.1016/j.jacc.2019.08.1036] [PMID]
28. Davoudi Kongsofla M, Najafi Ghezeljeh T, Saeidi A, Peyravi H, Kiaroosta N. Design and evaluation of a smartphone-based application to manage the treatment of people with heart failure. Iran J War Public Health. 2019;11(3):125-31. [Persian] [Link] [DOI:10.29252/ijwph.11.3.125]
29. Aghayousefi A, Amirpour B, Alipour A, Zare H. Effect of cognitive processing therapy on cardiovascular biomarkers of veterans with post-traumatic stress disorder. Iran J War Public Health. 2015;7(1):43-8. [Persian] [Link]








