Volume 13, Issue 2 (2021)
Iran J War Public Health 2021, 13(2): 155-162 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2021/08/29 | Accepted: 2021/08/30 | Published: 2021/11/1
Received: 2021/08/29 | Accepted: 2021/08/30 | Published: 2021/11/1
How to cite this article
AL-Taee S, AL-Jumaa Z, Al-Sarraj E, Hussein A, Abbas B. Fungemia and Fungal Diseases as Complication of COVID-19. Iran J War Public Health 2021; 13 (2) :155-162
URL: http://ijwph.ir/article-1-1005-en.html
URL: http://ijwph.ir/article-1-1005-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Pathology and Poultry Diseases, College of Veterinary Medicine, University of Mosul, Mosul, Iraq
2- Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Mosul, Mosul, Iraq
3- Department of Pharmacy, Al-Noor University College, Mosul, Iraq
4- Department of Anatomy and Histology, College of Veterinary Medicine, University of Basrah, Basrah, Iraq
5- Department of Microbiology, College of Veterinary Medicine, University of Basrah, Basrah, Iraq
2- Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Mosul, Mosul, Iraq
3- Department of Pharmacy, Al-Noor University College, Mosul, Iraq
4- Department of Anatomy and Histology, College of Veterinary Medicine, University of Basrah, Basrah, Iraq
5- Department of Microbiology, College of Veterinary Medicine, University of Basrah, Basrah, Iraq
Full-Text (HTML) (656 Views)
Introduction
Coronaviruses (CoVs) are single stranded and envelop RNA related to Coronaviridae family, order Nidovirales belong to sub-family: Orthocoronavirinae [1]. Sporadic and outbreak infections occur in both animals and humans as SARS-1, MERS which transmitted from animal to humans, recently pandemic SARS-2 has also revisable transmitting from human to pet animals [2]. In December 2019, SARS-2 was detected in Wuhan Seafood Market as a novel disease characterized by pneumonia with severe acute respiratory syndrome [3]. Because SARS-2 is a rapidly transmissible disease, there were about 10 million known cases and 500000 deaths in the first six months after the disease was discovered, and some cases associated with acute respiratory distress syndromes (ARDS) [4, 5]. This review aimed to identify the main fungal agent and explain the pathogenesis and pathological aspect as complicated to COVID-19.
Pathophysiological Mechanisms
COVID-19 primarily affects the respiratory and immunological systems, but it also affects other systems such as the urinary, gastrointestinal and reproductive tracts with neural and cardiovascular systems [6]. The spike protein is the main viral structural envelop for pathogenesis in the human, which binds to a human cell surface receptor protein called Angiotensin Converting Enzyme-2 (hACE2) via its receptor-binding domain (RBD). It is proteolytically activated by human proteases; also, cell entry of COVID-19 is reactivated by proprotein convertase furin, reducing its dependence on target cell proteases for entry [7] (Figure 1).

Figure 1) Host cellular proteases may trigger coronavirus spikes at various phases of coronavirus penetration [7]
SARS-cell CoVs entrance mechanism has been extensively researched. The receptor-binding domain (RBD) of SARS-CoV S1 bind to angiotensin-converting enzyme 2 (ACE2) as the virus's receptor [8]. One of the defense body mechanisms is a chemokine response and cytokine, which play a vital role in viral permission, whereas a dysregulated response might have disastrous consequences for infected patients [9]. The recruitment of hyperactive cytokines and chemokines causes a significant number of immune cells, such as macrophages, neutrophils, monocytes, and lymphocytes, to migrate from the bloodstream to the infection site
[10], deregulated cytokines and chemokines have been caused by sepsis and pathological changes in different variable organs as in (Figure 2).

Figure 2) Pathogenesis of SARS-2, cytokine storm and inflammatory injury to vital organs [11]
COVID-19 Coinfection
SARS-CoV-2 has far outperformed any other common flu virus in terms of infection and fatality since its discovery [12]; the coinfection of SARS-CoV-2 with other microbes, such as viruses, bacteria, and fungi, plays a major role in COVID-19, which is called "Superinfection". Superinfections caused by bacteria and fungi that are complicated as COVID-19 are more common in critically sick hospitalized patients with underlying systemic disorders, immunosuppression, overdose, prolonged treatment with a corticosteroid, mechanical ventilation, prolonged hospital, and ICU stay, and advanced age. Superinfections can exacerbate coronavirus illness in an immunocompetent host [13].
Mixed fungal coinfection maybe occur as Aspergillus fumigatus with a Mucorales species and causes several clinical forms, the hypothesis for the infection are some risk factors as usage steroid drug for treatment SARS-2 may act important roles for the development of molds, providing a good environment for colonizing the patient, SARS-CoV-2 infection itself considered as an immunosuppressive state [14].
Superinfection Pathophysiology Mechanisms
Mechanical and immunological factors damage the respiratory tract's host defenses after viral infection, exposing the patient to bacterial and fungal infection [15]; pathophysiology Mechanisms involve two predisposing factors:
Mechanical ventilation: Reports suggested that, predominantly from China, secondary infections were observed in 5–27% of SARS-CoV-2 infected individuals in multiple institutions, with 50–100% of those who died [16]. Patients with severe disease who are intensive care getting mechanical ventilation are more likely to get these infections.
-Dysregulation of the immune system of the host: The interaction between the virus and the host cell triggers an immune response, resulting in the production of anti-inflammatory cytokines like IL-4 and IL-10 as well as pro-inflammatory cytokines as IL-6, IL-2, and TNF-alpha; all these cytokines considered harmful and damage to host cells. Also, prolonged and severe antimicrobial administration may have a role in superinfection and die due to synergic effects or interaction between them [17]. A 30-year-old patient with pneumonia caused by Staphylococcus aureus, complicated with COVID-19, died after several days of treatment by clindamycin plus oxacillin and azithromycin for COVID-19, which was later changed to piperacillin-tazobactam and linezolid and then to meropenem, gentamicin, and linezolid; also an immunocompetent drug like corticosteroids have an important role in superinfection. Superinfections occur in many categories, as in Table 1.
Table 1) Categories and percentage of superinfections occurrence

Fungal Superinfection
Cases of COVID-19-induced "black fungus" are growing in some areas as the second wave of COVID-19. Aspergillosis, mucormycosis, and candidiasis infections produced by melanized fungus are examples of opportunistic mycoses that cause a wide spectrum of diseases. These affected patients take steroids and are immunocompromised. from localized infections to lethal disseminated disorders; some are transmitted from animal to human or indirect transmission from the environment as an endemic infection [18].
In COVID-19 patients with predisposing conditions, the incidence of opportunistic fungal infections is significantly higher (e.g., mechanical ventilation, cytokine storm, and diabetes). The majority of fungal infections in this group of patients, on the other hand, are related to the COVID-19 patients' difficult medical situations and the improper collection of clinical specimens. The respiratory tract represented the main target organs for COVID-19 infection and the most vital organs affected by super fungal infections (Figure 3).

Figure 3) The SARS-CoV-2, which is represented by black spiky circles and the coinfection fungal genera of the affected
patient with COVID-19, SARS-CoV-2 is represented by black spiky circles [25]
Mucormycosis (Black fungal disease) is a very uncommon infection. Mucor mold is widely distributed in plants, manure plants, and decaying vegetables and fruits. It is global and originates in air and soil, even in healthy people's noses and mucous. Mucormycosis, which has an overall mortality rate of 50%, may be triggered by steroids.
Steroids reduce inflammation in the lungs for COVID-19 and appear to help stop some of the damage that can happen when the body's immune system goes into overdrive to fight off coronavirus. But they also reduce immunity and push up blood sugar levels in people with diabetes and non-diabetic COVID-19 patients [26].
Mucormycosis hyphal angeo-invasive fungal infection associated with a high rate of morbidity and mortality, the brain, sinuses, and
paranasal tissue are the main target organs; however, it can also affect the heart, spleen, and skin, as in table [2, 27, 28].
Table 2) Mucormycosis types and target organs for infection in the most susceptible patients

Mucormycosis is not a contagious fungal that is transmitted through (i) inhaled spores and invade the lung and sinus, (ii) affected the skin if the infection occurs through a bite, burn. The infection then directly through circulation spreads to internal organs involve the eye, brain, spleen, heart, different pathways of causative agent entry lead to differences in clinical signs [29]. In general, atypical clinical signs take about four weeks, characterized by the nasal blockade, proptosis, crusting, edema, and facial swelling, painful necrotic skin (Figure 4), headache and fever, even chemosis, and ptosis periorbital inflammation with ophthalmoplegia with neurological signs [30, 31]. Histopathological examination revealed paranasal sinus tissue necrosis, angio-invasive, and vasculitis with granulomatous inflammation [23, 32].
Figure 4) Fascial swelling and necrosis (A), necrotic debris in the paranasal sinus tissue with vasculitis (B), with granulomatous inflammation (C); Hematoxylin and eosin 200X [23, 31]
Invasive Pulmonary Aspergillosis (IPA) is another fungal disease that occurs as coinfection with COVID-19 because of lung damage and lowers respiratory tract, mainly occurring in patients with neutropenia and prolonged treatment with prolonged treatment corticosteroid [33]. In Wuhan, the mortality rate in patients with COVID-19 reached approximately 60–64.7% due to mechanical ventilator and invasive fungal A. fumigatus, which is reported as mainly coinfection and more common fungal prevalence as 70% from cases with fungal coinfection occurs with pandemic COVID-19 in the world wide country [34-36].
Cytokines Releasing Syndrome (CRS) associated with IPA: In severe COVID-19 patients, pro-inflammatory chemokines and cytokines such as TNFa, monocyte chemoattractant protein-1, interleukin-1b, IL-6, and IL-10 were greatly enhanced. The raised cytokine levels called (storm cytokines) may also play a role in COVID-19's deadly consequences; the histopathological examination revealed interstitial infiltrations with macrophage and monocyte in the heart, lung, and gastrointestinal mucosa and tissue necrosis in severe COVID-19 patients with high inflammatory cytokines [37]. IL-10 is one of the most important cytokines, which is considered a key for many immune responses. It mediates macrophage activity and limits the level of local tissue destruction. In the COVID-19, there is a greater activity of T helper cells (Th2) with increased levels of IL-10, combined with a depression in the Th1 that causes depression in the macrophage activity and elevates host susceptibility Aspergillus infection [38].
Also, IL-6 is one of the CRS that has a role in a blockage targeting the host immune system that may be effective for COVID-19. However, at the same time, it causes biological damage as disturbances in vessel permeability, cardiac arrhythmia, and reducing myocardium contractility and acute respiratory distress syndrome (ARDS) and increases susceptibility to IPA at this case, the T cells' reactivity to IL-6 is diminished [39, 40].
A. fumigatus isolated from patients a Broncho-alveolar lavage (BAL), and is characterized by septate hyphae with fruiting head and broad hyaline branching (Figure 5) and cause bilateral pneumonia and follicular lymphoma [41].
-Candidiasis invasive candidiasis is a serious healthcare-associated fungal infection that causes significant mortality rates. It is caused by various opportunistic Candida species, the most frequent Candida albicans and C. glabrata [42]. According to data from a Spanish hospital, invasive candidiasis is becoming more common among COVID-19-positive patients with a higher fatality rate. During COVID-19 pandemic events in New York City, USA, Candida spp. was one of the most commonly detected fungi in the bloodstream (candidemia) of patients with central venous catheters; Candida species were also isolated from oropharyngeal which cause damage to the epithelial cells [43, 44].

Figure 5) A: A. fumigatus characterized by septate hyphae with fruiting head (Lactophenol cotton blue, 200X); B: broad hyaline branching and cause bilateral pneumonia and follicular lymphoma (Hematoxylin and eosin, 200X), [41].
On the other hand, Saccharomyces organisms have been described as invasive infection agents in malignance or immunocompromised patients following probiotic treatment with Saccharomyces cerevisiae, which was detected in two patients hospitalized in the ICU due to severe COVID-19 [45]. Fungaemias is the translocation of the yeast to the blood circulation through mucositis and ulcers; another portal entry is through the central venous catheter [46, 47]. Yeast overgrowth and gastrointestinal (GIT) escape, produced by direct or indirect GIT damage, maybe key pathogenic factors for invasive mycoses. Haemodialysis, intestinal surgery and severe chemotherapy all these risk factors play a part in the GI leakage and lead to Sepsis-Related Organ Failure Assessment score (SOFA score) and the gravity of the disease in terms of septic shock.
Coinfections with COVID-19 and Pneumocystis also have been reported. Pneumocystis jirovecii is an opportunistic pathogen and an atypical unicellular fungus. Pneumocystis can produce Pneumocystis pneumonia or pneumocystosis, and variable lesions in many organs in immunocompromised patients (due to HIV, cancer, immunosuppressive medication, organ donation, or congenital immunodeficiencies) [48] Pneumocystis pneumonia is a kind of pneumocystis [49]. Descript the Histopathological lesions in the variable organs so in the lung detected fibrosis and thickening of the alveolar septa, microcapillary thrombus with micro-hyaline –fibrin and/ or platelet thrombus in the alveolar-capillary in addition to exudate in the alveolar and may reach to the bronchial lumen with foamy macrophage (Figure 6). There was a hyper trophy of myofiber in the hearing, alcoholic steatosis is seen in the liver with hepatitis and extensive tubular necrosis in the kidney (shock kidney), and significant medullary hemorrhage in the adrenal glands (Figure 7).

Figure 6) Histopathological examination of the lung revealed fibrosis and thickening of the alveolar septa (A) x100, microcapillary thrombus (B) x40, with micro-hyaline –fibrin (C) x200and/ or platelet thrombus in the alveolar-capillary (D) x100in addition to exudate in the alveolar (E) x400 and may reach to the bronchial lumen with foamy macrophage (F)x200; Hematoxylin and Eosin [49]
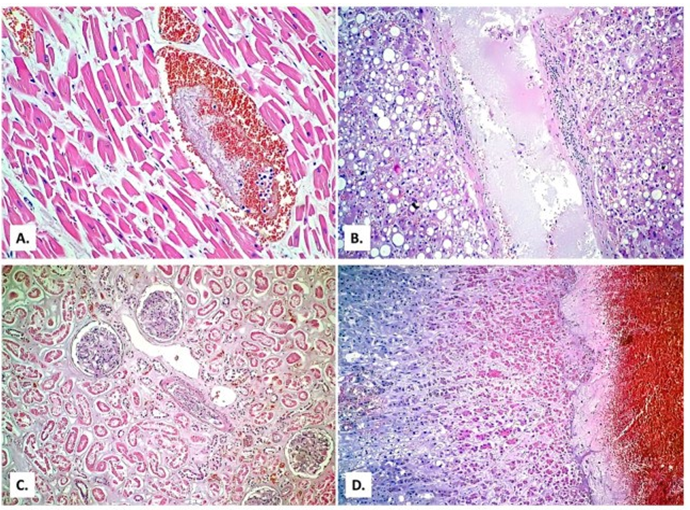
Figure 7) Histopathological examination In the hearing, there was hypertrophy of myofiber (A) × 400, alcoholic steatosis is seen in the liver with hepatitis (B) x 100 and extensive tubular necrosis in the kidney (shock kidney) (C) x200, as well as significant medullaryhemorrhage in the adrenal glands (D) x40; Hematoxylin and eosin [49]
Conclusion
The high rate of severe infection and mortality in patients with COVID-19's is thought to be due in part to a lack of natural immunity and raped viral replication in the lower respiratory tract, as well as superinfections, secondary infections, or co-infections, mainly fungal agent that cause severe lung injury and acute respiratory distress syndrome (ARDS) as well as cause damage and sepsis in other organs.
Acknowledgments: Authors did not declare this item.
Ethical Permissions: Authors did not declare this item.
Conflicts of Interests: Authors did not declare this item.
Authors’ Contribution: AL-Taee Sh.K. (First Author), Introduction Writer/Methodologist/Main Researcher (20%); AL-Jumaa Z.M. (Second Author), Introduction Writer/Methodologist (20%); Al-Sarraj E.S.Y. (Third Author), Assistant Researcher (20%); Hussein A.J. (Forth Author), Assistant Researcher (20%); Abbas B.A. (Fifth author), Assistant Researcher (20%).
Funding/Support: Authors did not declare this item.
Coronaviruses (CoVs) are single stranded and envelop RNA related to Coronaviridae family, order Nidovirales belong to sub-family: Orthocoronavirinae [1]. Sporadic and outbreak infections occur in both animals and humans as SARS-1, MERS which transmitted from animal to humans, recently pandemic SARS-2 has also revisable transmitting from human to pet animals [2]. In December 2019, SARS-2 was detected in Wuhan Seafood Market as a novel disease characterized by pneumonia with severe acute respiratory syndrome [3]. Because SARS-2 is a rapidly transmissible disease, there were about 10 million known cases and 500000 deaths in the first six months after the disease was discovered, and some cases associated with acute respiratory distress syndromes (ARDS) [4, 5]. This review aimed to identify the main fungal agent and explain the pathogenesis and pathological aspect as complicated to COVID-19.
Pathophysiological Mechanisms
COVID-19 primarily affects the respiratory and immunological systems, but it also affects other systems such as the urinary, gastrointestinal and reproductive tracts with neural and cardiovascular systems [6]. The spike protein is the main viral structural envelop for pathogenesis in the human, which binds to a human cell surface receptor protein called Angiotensin Converting Enzyme-2 (hACE2) via its receptor-binding domain (RBD). It is proteolytically activated by human proteases; also, cell entry of COVID-19 is reactivated by proprotein convertase furin, reducing its dependence on target cell proteases for entry [7] (Figure 1).

Figure 1) Host cellular proteases may trigger coronavirus spikes at various phases of coronavirus penetration [7]
SARS-cell CoVs entrance mechanism has been extensively researched. The receptor-binding domain (RBD) of SARS-CoV S1 bind to angiotensin-converting enzyme 2 (ACE2) as the virus's receptor [8]. One of the defense body mechanisms is a chemokine response and cytokine, which play a vital role in viral permission, whereas a dysregulated response might have disastrous consequences for infected patients [9]. The recruitment of hyperactive cytokines and chemokines causes a significant number of immune cells, such as macrophages, neutrophils, monocytes, and lymphocytes, to migrate from the bloodstream to the infection site
[10], deregulated cytokines and chemokines have been caused by sepsis and pathological changes in different variable organs as in (Figure 2).

Figure 2) Pathogenesis of SARS-2, cytokine storm and inflammatory injury to vital organs [11]
COVID-19 Coinfection
SARS-CoV-2 has far outperformed any other common flu virus in terms of infection and fatality since its discovery [12]; the coinfection of SARS-CoV-2 with other microbes, such as viruses, bacteria, and fungi, plays a major role in COVID-19, which is called "Superinfection". Superinfections caused by bacteria and fungi that are complicated as COVID-19 are more common in critically sick hospitalized patients with underlying systemic disorders, immunosuppression, overdose, prolonged treatment with a corticosteroid, mechanical ventilation, prolonged hospital, and ICU stay, and advanced age. Superinfections can exacerbate coronavirus illness in an immunocompetent host [13].
Mixed fungal coinfection maybe occur as Aspergillus fumigatus with a Mucorales species and causes several clinical forms, the hypothesis for the infection are some risk factors as usage steroid drug for treatment SARS-2 may act important roles for the development of molds, providing a good environment for colonizing the patient, SARS-CoV-2 infection itself considered as an immunosuppressive state [14].
Superinfection Pathophysiology Mechanisms
Mechanical and immunological factors damage the respiratory tract's host defenses after viral infection, exposing the patient to bacterial and fungal infection [15]; pathophysiology Mechanisms involve two predisposing factors:
Mechanical ventilation: Reports suggested that, predominantly from China, secondary infections were observed in 5–27% of SARS-CoV-2 infected individuals in multiple institutions, with 50–100% of those who died [16]. Patients with severe disease who are intensive care getting mechanical ventilation are more likely to get these infections.
-Dysregulation of the immune system of the host: The interaction between the virus and the host cell triggers an immune response, resulting in the production of anti-inflammatory cytokines like IL-4 and IL-10 as well as pro-inflammatory cytokines as IL-6, IL-2, and TNF-alpha; all these cytokines considered harmful and damage to host cells. Also, prolonged and severe antimicrobial administration may have a role in superinfection and die due to synergic effects or interaction between them [17]. A 30-year-old patient with pneumonia caused by Staphylococcus aureus, complicated with COVID-19, died after several days of treatment by clindamycin plus oxacillin and azithromycin for COVID-19, which was later changed to piperacillin-tazobactam and linezolid and then to meropenem, gentamicin, and linezolid; also an immunocompetent drug like corticosteroids have an important role in superinfection. Superinfections occur in many categories, as in Table 1.
Table 1) Categories and percentage of superinfections occurrence

Fungal Superinfection
Cases of COVID-19-induced "black fungus" are growing in some areas as the second wave of COVID-19. Aspergillosis, mucormycosis, and candidiasis infections produced by melanized fungus are examples of opportunistic mycoses that cause a wide spectrum of diseases. These affected patients take steroids and are immunocompromised. from localized infections to lethal disseminated disorders; some are transmitted from animal to human or indirect transmission from the environment as an endemic infection [18].
In COVID-19 patients with predisposing conditions, the incidence of opportunistic fungal infections is significantly higher (e.g., mechanical ventilation, cytokine storm, and diabetes). The majority of fungal infections in this group of patients, on the other hand, are related to the COVID-19 patients' difficult medical situations and the improper collection of clinical specimens. The respiratory tract represented the main target organs for COVID-19 infection and the most vital organs affected by super fungal infections (Figure 3).

Figure 3) The SARS-CoV-2, which is represented by black spiky circles and the coinfection fungal genera of the affected
patient with COVID-19, SARS-CoV-2 is represented by black spiky circles [25]
Mucormycosis (Black fungal disease) is a very uncommon infection. Mucor mold is widely distributed in plants, manure plants, and decaying vegetables and fruits. It is global and originates in air and soil, even in healthy people's noses and mucous. Mucormycosis, which has an overall mortality rate of 50%, may be triggered by steroids.
Steroids reduce inflammation in the lungs for COVID-19 and appear to help stop some of the damage that can happen when the body's immune system goes into overdrive to fight off coronavirus. But they also reduce immunity and push up blood sugar levels in people with diabetes and non-diabetic COVID-19 patients [26].
Mucormycosis hyphal angeo-invasive fungal infection associated with a high rate of morbidity and mortality, the brain, sinuses, and
paranasal tissue are the main target organs; however, it can also affect the heart, spleen, and skin, as in table [2, 27, 28].
Table 2) Mucormycosis types and target organs for infection in the most susceptible patients

Mucormycosis is not a contagious fungal that is transmitted through (i) inhaled spores and invade the lung and sinus, (ii) affected the skin if the infection occurs through a bite, burn. The infection then directly through circulation spreads to internal organs involve the eye, brain, spleen, heart, different pathways of causative agent entry lead to differences in clinical signs [29]. In general, atypical clinical signs take about four weeks, characterized by the nasal blockade, proptosis, crusting, edema, and facial swelling, painful necrotic skin (Figure 4), headache and fever, even chemosis, and ptosis periorbital inflammation with ophthalmoplegia with neurological signs [30, 31]. Histopathological examination revealed paranasal sinus tissue necrosis, angio-invasive, and vasculitis with granulomatous inflammation [23, 32].
Figure 4) Fascial swelling and necrosis (A), necrotic debris in the paranasal sinus tissue with vasculitis (B), with granulomatous inflammation (C); Hematoxylin and eosin 200X [23, 31]
Invasive Pulmonary Aspergillosis (IPA) is another fungal disease that occurs as coinfection with COVID-19 because of lung damage and lowers respiratory tract, mainly occurring in patients with neutropenia and prolonged treatment with prolonged treatment corticosteroid [33]. In Wuhan, the mortality rate in patients with COVID-19 reached approximately 60–64.7% due to mechanical ventilator and invasive fungal A. fumigatus, which is reported as mainly coinfection and more common fungal prevalence as 70% from cases with fungal coinfection occurs with pandemic COVID-19 in the world wide country [34-36].
Cytokines Releasing Syndrome (CRS) associated with IPA: In severe COVID-19 patients, pro-inflammatory chemokines and cytokines such as TNFa, monocyte chemoattractant protein-1, interleukin-1b, IL-6, and IL-10 were greatly enhanced. The raised cytokine levels called (storm cytokines) may also play a role in COVID-19's deadly consequences; the histopathological examination revealed interstitial infiltrations with macrophage and monocyte in the heart, lung, and gastrointestinal mucosa and tissue necrosis in severe COVID-19 patients with high inflammatory cytokines [37]. IL-10 is one of the most important cytokines, which is considered a key for many immune responses. It mediates macrophage activity and limits the level of local tissue destruction. In the COVID-19, there is a greater activity of T helper cells (Th2) with increased levels of IL-10, combined with a depression in the Th1 that causes depression in the macrophage activity and elevates host susceptibility Aspergillus infection [38].
Also, IL-6 is one of the CRS that has a role in a blockage targeting the host immune system that may be effective for COVID-19. However, at the same time, it causes biological damage as disturbances in vessel permeability, cardiac arrhythmia, and reducing myocardium contractility and acute respiratory distress syndrome (ARDS) and increases susceptibility to IPA at this case, the T cells' reactivity to IL-6 is diminished [39, 40].
A. fumigatus isolated from patients a Broncho-alveolar lavage (BAL), and is characterized by septate hyphae with fruiting head and broad hyaline branching (Figure 5) and cause bilateral pneumonia and follicular lymphoma [41].
-Candidiasis invasive candidiasis is a serious healthcare-associated fungal infection that causes significant mortality rates. It is caused by various opportunistic Candida species, the most frequent Candida albicans and C. glabrata [42]. According to data from a Spanish hospital, invasive candidiasis is becoming more common among COVID-19-positive patients with a higher fatality rate. During COVID-19 pandemic events in New York City, USA, Candida spp. was one of the most commonly detected fungi in the bloodstream (candidemia) of patients with central venous catheters; Candida species were also isolated from oropharyngeal which cause damage to the epithelial cells [43, 44].

Figure 5) A: A. fumigatus characterized by septate hyphae with fruiting head (Lactophenol cotton blue, 200X); B: broad hyaline branching and cause bilateral pneumonia and follicular lymphoma (Hematoxylin and eosin, 200X), [41].
On the other hand, Saccharomyces organisms have been described as invasive infection agents in malignance or immunocompromised patients following probiotic treatment with Saccharomyces cerevisiae, which was detected in two patients hospitalized in the ICU due to severe COVID-19 [45]. Fungaemias is the translocation of the yeast to the blood circulation through mucositis and ulcers; another portal entry is through the central venous catheter [46, 47]. Yeast overgrowth and gastrointestinal (GIT) escape, produced by direct or indirect GIT damage, maybe key pathogenic factors for invasive mycoses. Haemodialysis, intestinal surgery and severe chemotherapy all these risk factors play a part in the GI leakage and lead to Sepsis-Related Organ Failure Assessment score (SOFA score) and the gravity of the disease in terms of septic shock.
Coinfections with COVID-19 and Pneumocystis also have been reported. Pneumocystis jirovecii is an opportunistic pathogen and an atypical unicellular fungus. Pneumocystis can produce Pneumocystis pneumonia or pneumocystosis, and variable lesions in many organs in immunocompromised patients (due to HIV, cancer, immunosuppressive medication, organ donation, or congenital immunodeficiencies) [48] Pneumocystis pneumonia is a kind of pneumocystis [49]. Descript the Histopathological lesions in the variable organs so in the lung detected fibrosis and thickening of the alveolar septa, microcapillary thrombus with micro-hyaline –fibrin and/ or platelet thrombus in the alveolar-capillary in addition to exudate in the alveolar and may reach to the bronchial lumen with foamy macrophage (Figure 6). There was a hyper trophy of myofiber in the hearing, alcoholic steatosis is seen in the liver with hepatitis and extensive tubular necrosis in the kidney (shock kidney), and significant medullary hemorrhage in the adrenal glands (Figure 7).

Figure 6) Histopathological examination of the lung revealed fibrosis and thickening of the alveolar septa (A) x100, microcapillary thrombus (B) x40, with micro-hyaline –fibrin (C) x200and/ or platelet thrombus in the alveolar-capillary (D) x100in addition to exudate in the alveolar (E) x400 and may reach to the bronchial lumen with foamy macrophage (F)x200; Hematoxylin and Eosin [49]

Figure 7) Histopathological examination In the hearing, there was hypertrophy of myofiber (A) × 400, alcoholic steatosis is seen in the liver with hepatitis (B) x 100 and extensive tubular necrosis in the kidney (shock kidney) (C) x200, as well as significant medullaryhemorrhage in the adrenal glands (D) x40; Hematoxylin and eosin [49]
Conclusion
The high rate of severe infection and mortality in patients with COVID-19's is thought to be due in part to a lack of natural immunity and raped viral replication in the lower respiratory tract, as well as superinfections, secondary infections, or co-infections, mainly fungal agent that cause severe lung injury and acute respiratory distress syndrome (ARDS) as well as cause damage and sepsis in other organs.
Acknowledgments: Authors did not declare this item.
Ethical Permissions: Authors did not declare this item.
Conflicts of Interests: Authors did not declare this item.
Authors’ Contribution: AL-Taee Sh.K. (First Author), Introduction Writer/Methodologist/Main Researcher (20%); AL-Jumaa Z.M. (Second Author), Introduction Writer/Methodologist (20%); Al-Sarraj E.S.Y. (Third Author), Assistant Researcher (20%); Hussein A.J. (Forth Author), Assistant Researcher (20%); Abbas B.A. (Fifth author), Assistant Researcher (20%).
Funding/Support: Authors did not declare this item.
References
1. International committee on taxonomy of viruses. Coronaviridea [Internet]. Moscow: ICTV; 2011 [cited 2020 May 25]. Available from: https://talk.ictvonline.org/ictv-reports/ictv_9th_report/positive-sense-rna-viruses-2011/w/posrna_viruses/222/coronaviridae. [Link]
2. AL-Taee SK, AL-Jumaa ZM, Ali FF. Coronavirus and COVID-19 disease in aquatic animals' aspects. Vet Pract. 2020;21(1):107-12. [Link]
3. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-207. [Link] [DOI:10.1056/NEJMoa2001316] [PMID] [PMCID]
4. WHO. Coronavirus disease (COVID-19) [Internet]. Geneva: WHO; 2020 [cited 2020 Jun 30]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. [Link]
5. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. [Link] [DOI:10.1016/S0140-6736(20)30183-5]
6. Deshmukh V, Motwani R, Kumar A, Kumari C, Raza K. Histopathological observations in COVID-19: A systematic review. J Clin Pathol. 2021;74(2):76-83. [Link] [DOI:10.1136/jclinpath-2020-206995] [PMID]
7. Shanga J, Wana Y, Luoa C, Yea G, Genga Q, Auerbacha A, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci. 2020;117(21):11727-34. [Link] [DOI:10.1073/pnas.2003138117] [PMID] [PMCID]
8. Li F. Receptor recognition and cross-species infections of SARS coronavirus. Antiviral Res. 2013;100(1):246-54. [Link] [DOI:10.1016/j.antiviral.2013.08.014] [PMID] [PMCID]
9. Gralinski LE, Baric RS. Molecular pathology of emerging coronavirus infections. J Pathol. 2015;235(2):185-95. [Link] [DOI:10.1002/path.4454] [PMID] [PMCID]
10. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529-39. [Link] [DOI:10.1007/s00281-017-0629-x] [PMID] [PMCID]
11. Zhu Z, Lian X, Su X, Wu W, Marraro GA, Zeng Y. From SARS and MERS to COVID-19: A brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir Res. 2020;21(1):224. [Link] [DOI:10.1186/s12931-020-01479-w] [PMID] [PMCID]
12. Li R, Pei S, Chen B, Song Y, Zhang T, Yang W, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science. 2020;368(6490):489-93. [Link] [DOI:10.1126/science.abb3221] [PMID] [PMCID]
13. Blaize M, Mayaux J, Nabet C, Lampros A, Marcelin A, Thellier M, et al. Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis. 2020;26(7):1636-7. [Link] [DOI:10.3201/eid2607.201603] [PMID] [PMCID]
14. Pasero D, Sanna S, Liperi C, Piredda D, Branca GP, Casadio L, et al. A challenging complication following SARS-CoV-2 infection: A case of pulmonary mucormycosis. Infection. 2020 Dec:1-6. [Link] [DOI:10.1007/s15010-020-01561-x] [PMID] [PMCID]
15. Alp E, Voss A. Ventilator associated pneumonia and infection control. Ann Clin Microbiol Antimicrob. 2006;5:7-1. [Link] [DOI:10.1186/1476-0711-5-7] [PMID] [PMCID]
16. Pedersen SF, Ho YC. SARS-CoV-2: A storm is raging. J Clin Invest. 2020;130(5):2202-5. [Link] [DOI:10.1172/JCI137647] [PMID] [PMCID]
17. Duployez C, Le Guern R, Tinez C, Lejeune AL, Robriquet L, Six S, et al. Panton-valentine leukocidin-secreting staphylococcus aureus pneumonia complicating COVID-19. Emerg Infect Dis. 2020;26(8):1939-41. [Link] [DOI:10.3201/eid2608.201413] [PMID] [PMCID]
18. Seyedmousavi S, Bosco SMG, De Hoog S, Ebel F, Elad D, et al. Fungal infections in animals: A patchwork of different situations. Med Mycol. 2018;56(Suppl 1):165-87. [Link] [DOI:10.1093/mmy/myx104] [PMID] [PMCID]
19. Sepulveda J, Westblade LF, Whittier S, Satlin MJ, Greendyke WG, Aaron JG, et al. Bacteremia and blood culture utilization during COVID-19 surge in New York city. J Clin Microbiol. 2020;58(8):00875-20. [Link] [DOI:10.1128/JCM.00875-20]
20. Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-81. [Link] [DOI:10.1016/S2213-2600(20)30079-5]
21. He Y, Li W, Wang Z, Chen H, Tian L, Liu D. Nosocomial infection among patients with COVID-19: A retrospective data analysis of 918 cases from a single center in Wuhan, China. Infect Control Hosp Epidemiol. 2020;41(5):982-3. [Link] [DOI:10.1017/ice.2020.126] [PMID] [PMCID]
22. International society for infectious disease. Guide to infection control in the healthcare setting [Internet]. New York: International Society for Infectious Disease; 2018 [cited: Unknown]. Avaialable from: https://isid.org/guide/. [Link]
23. Veisi A, Bagheri A, Eshaghi M, Rikhtehgar MH, Kanavi MR, Farjad R. Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: A case report. Eur J Ophthalmol. 2021 Mar:1-6. [Link] [DOI:10.1177/11206721211009450] [PMID]
24. Buil JB, Meijer EFJ, Denning DW, Verweij PE, Meis JF. Burden of serious fungal infections in the Netherlands. Mycoses. 2020;63(6):625-31. [Link] [DOI:10.1111/myc.13089] [PMID] [PMCID]
25. Silva LN, De Mello TP, De Souza Ramos L, Branquinha MH, Roudbary M, Dos Santos ALS. Fungal infections in COVID-19-positive patients: A lack of optimal treatment options. Curr Top Med Chem. 2020;20(22):1951-7. [Link] [DOI:10.2174/156802662022200917110102] [PMID]
26. Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: A deadly addition to the pandemic spectrum. J Laryngol Otol. to the pandemic spectrum. 2021;135(5):442-7. [Link] [DOI:10.1017/S0022215121000992] [PMID] [PMCID]
27. Francis JR, Villanueva P, Bryant P, Blyth CC. Mucormycosis in children: Review and recommendations for management. J Pediatr Infect Dis Soc. 2018;7(2):159-64. [Link] [DOI:10.1093/jpids/pix107] [PMID]
28. Song Y, Qiao J, Giovanni G, Liu G, Yang H, Wu J, et al. Mucormycosis in renal transplant recipients: Review of 174 reported cases. BMC Infect Dis. 2017;17:283. [Link] [DOI:10.1186/s12879-017-2381-1] [PMID] [PMCID]
29. Bhat I, Beg MA, Athar F. A contemporary intimidation for COVID-19 patients coinfected with mucormycosis in India. J Bacteriol Mycol Open Access. 2021;9(2):69-71. [Link] [DOI:10.15406/jbmoa.2021.09.00298]
30. Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi. 2019;5(1):26. [Link] [DOI:10.3390/jof5010026] [PMID] [PMCID]
31. Scheckenbach K, Cornely O, Hoffmann TK, Engers R, Bier H, Chaker A, et al. Emerging therapeutic options in fulminant invasive rhinocerebral mucormycosis. Auris Nasus Larynx. 2010;37(3):322-8. [Link] [DOI:10.1016/j.anl.2009.09.001] [PMID]
32. Razem B, Dennai Y, Slimani F. Chronical rhino-orbital mucormycosis in an immunocompetent host: A case report. Int J Surg Case Rep. 2021;82:105882. [Link] [DOI:10.1016/j.ijscr.2021.105882] [PMID] [PMCID]
33. Kwon WJ, Zheng M, Kaur H, Magbual N, Dalai S. Superinfections and coinfections in COVID-19-separating the signal from the noise [Internet]. Unknown Publisher City: Medpage Today; 2020 (cited 2020 Jun 21). Available from: [Link]
34. Lai CC, Yu WL. COVID-19 associated with pulmonary aspergillosis: A literature review. J Microbiol Immunol Infect. 2021;54(1):46-53. [Link] [DOI:10.1016/j.jmii.2020.09.004] [PMID] [PMCID]
35. Ezeokoli OT, Pohl CH. Opportunistic pathogenic fungal coinfections are prevalent in critically ill COVID-19 patients: Are they risk factors for disease severity. S Afr Med J. 2020;110(11):1081-5. [Link] [DOI:10.7196/SAMJ.2020.v110i11.15248]
36. Falcone M, Massetti AP, Russo A, Vullo V, Venditti M. Invasive aspergillosis in patients with liver disease. Med Mycol. 2011;49(4):406-13. [Link] [DOI:10.3109/13693786.2010.535030] [PMID]
37. Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-Mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35(3):266-71. [Link] [DOI:10.1007/s12250-020-00207-4] [PMID] [PMCID]
38. Clemons KV, Grunig G, Sobel RA, Mirels LF, Rennick DM, Stevens DA. Role of IL-10 in invasive aspergillosis: Increased resistance of IL-10 gene knockout mice to lethal systemic aspergillosis. Clin Exp Immunol. 2000;122(2):186-91. [Link] [DOI:10.1046/j.1365-2249.2000.01382.x] [PMID] [PMCID]
39. Cai S, Sun W, Li M, Dong L. A complex COVID-19 case with rheumatoid arthritis treated with tocilizumab. Clin Rheumatol. 2020;39(2):2797-802. [Link] [DOI:10.1007/s10067-020-05234-w] [PMID] [PMCID]
40. Camargo JF, Bhimji A, Kumar D, Kaul R, Pavan R, Schuh A, et al. Impaired T cell responsiveness to interleukin-6 in hematological patients with invasive aspergillosis. Plos One. 2015;10(4):0123171. [Link] [DOI:10.1371/journal.pone.0123171] [PMID] [PMCID]
41. Johnson AK, Ghazarian Z, Cendrowski KD, Persichino JG. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med Mycol Case Rep. 2021;32:64-7. [Link] [DOI:10.1016/j.mmcr.2021.03.006] [PMID] [PMCID]
42. Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026. [Link] [DOI:10.1038/nrdp.2018.26] [PMID]
43. Nori P, Cowman K, Chen V, Bartash R, Szymczak W, Madaline T, et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York city pandemic surge. Infect Control Hosp Epidemiol. 2020;42(1):84-8. [Link] [DOI:10.1017/ice.2020.368] [PMID] [PMCID]
44. Vila T, Sultan AS, Montelongo-Jauregui D, Jabra-Rizk MA. Oral candidiasis: A disease of opportunity. J Fungi. 2020;6(1):15. [Link] [DOI:10.3390/jof6010015] [PMID] [PMCID]
45. Ventoulis I, Sarmourli T, Amoiridou P, Mantzana P, Exindari M, Gioula G, et al. Bloodstream infection by Saccharomyces cerevisiae in two COVID-19 patients after receiving supplementation of saccharomyces in the ICU. J Fungi. 2020;6(3):98. [Link] [DOI:10.3390/jof6030098] [PMID] [PMCID]
46. Sulik-Tyszka B, Snarski E, Nied'zwiedzka M, Augustyniak M, Myhre TN, Kacprzyk A, et al. Experience with saccharomyces boulardii probiotic in oncohaematological patients. Probiotics Antimicrob Proteins. 2018;10(2):350-5. [Link] [DOI:10.1007/s12602-017-9332-4] [PMID] [PMCID]
47. Cassone M, Serra P, Mondello F, Girolamo A, Scafetti S, Pistella E, et al. Outbreak of Saccharomyces cerevisiae subtype Boulardii Fungemia in patients neighboring those treated with a probiotic preparation of the organism. J. Clin Microbiol. 2003;41(11):5340-3. [Link] [DOI:10.1128/JCM.41.11.5340-5343.2003] [PMID] [PMCID]
48. De Armas Rodriguez Y, Wissmann G, Muller AL, Pederiva MA, Brum MC, Brackmann RL, et al. Pneumocystis jirovecii pneumonia in developing countries. Parasite. 2011;18(3):219-28. [Link] [DOI:10.1051/parasite/2011183219] [PMID] [PMCID]
49. Jeican II, Inisca P, Gheban D, Tabaran F, Aluas M, Trombitas V, et al. COVID-19 and Pneumocystis Jirovecii pulmonary coinfection-the first case confirmed through autopsy. Medicina. 2021;57(4):302. [Link] [DOI:10.3390/medicina57040302] [PMID] [PMCID]








